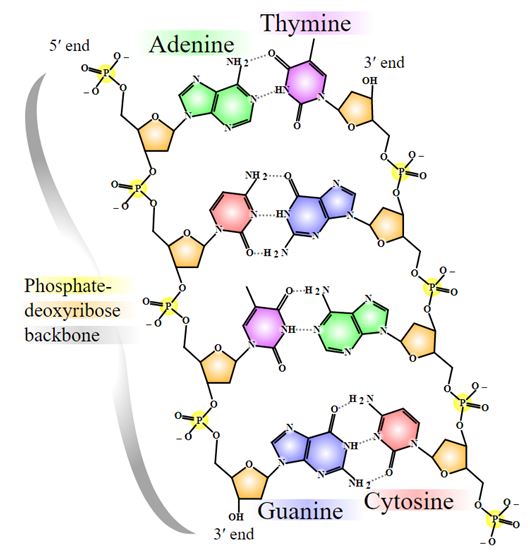
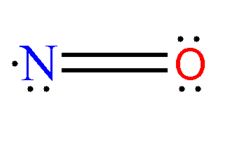Nevertheless the truly novel feature of the DNA structure, as Watson and Crick put it in their paper, is the way that the DNA strands (“ribbons”)are held together. Erwin Chargaff’s paper chromatograhy techniques had revealed that DNA’s content consistently included almost equal amounts of adenine(A) and thymine(T). The same was true of guanine(G) and cytosine(C). Crick and Watson realized that A bonded T while G held on to C; these pairings come about because they produce the most possible number of hydrogen bonds between these so-called purine and pyramidine ”bases”(Figure 14).
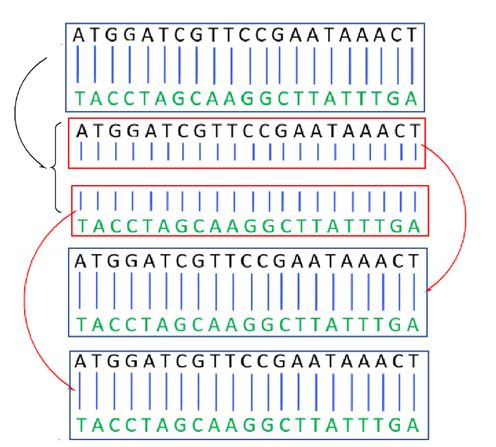
In cell division, the DNA molecule is slowly unwound, exposing A,T,G and C. If the complementary bases are then supplied to each half of the helix, two identical copies will emerge. This is what happens during cell division when each cell must first replicate its 46 chromosomes to create 92 before becoming two cells (Figure 15).
How did DNA transmit information? Recall that all life forms use 20 amino acids to make their highly versatile proteins. If the code for an amino acid consisted of only a single base, there would be only 4 codes, one for each of A, T, G and C. If there were two bases in the code, there would be enough codes for only 42 amino acids. Three bases is the way to go: with 43 = 64 possibilities, there could be at least one code per amino acid, and there is also room for stop codes, to tell the protein-making process when to terminate a chain of amino acids.
i. RNA
The workhorse-molecules who assemble the amino acids into polypeptides don’t read the codes directly from DNA. From select parts of a gene, the codes are transcribed into a simpler “foreman”-molecule known as messenger RNA(mRNA). mRNA is single-stranded molecule using ribose as its sugar in its untwisted spine. Another difference between mRNA and DNA is that any type of RNA uses the pyrimidine base uracil(U) instead of thymine, but the difference (an H attached to its ring instead of CH3) is almost negligent because it still makes 2 hydrogen bonds with adenine. mRNA has complementary bases to those of the usable parts of DNA. For example, if the DNA code from a gene includes …TACGGCATG…, the mRNA assembled will have …AUGCCGUAC….
On the ribosomes, the foreman will sit there while workers fetch the appropriate amino acid. The workers are another form of RNA known as transfer RNA (tRNA) . Each carries a triplet(three bases) and its structure is such that it only bonds to one amino acid. For example, a tRNA with the triplet UAC will only pick up methionine (Met). The tRNA, with its Met will bond temporarily to the mRNA at the right spot where the complementary bases match AUG(Figure 16a).
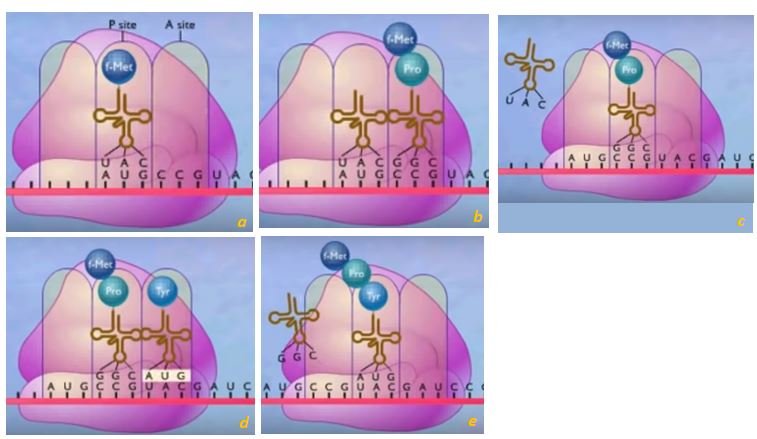
A tRNA carrying another amino acid will not have the appropriate triplet, will not bond there to mRNA and will not be bringing the wrong ingredient for the recipe. A tRNA with GGC will be carrying the amino acid proline(Pro) to the CCG spot on mRNA. Enzymes will form a peptide bond between the two amino acids, linking them together (Figure 16b). The entire assemblage is slid over by the space of a triplet, the UAC tRNA will be released so it could keep working(Figure 16 c), while a third triplet from mRNA (UAC) will await an ATG tRNA carrying tyrosine (Tyr).(Figures 16d, e) This goes on until a STOP signal is reached and the final polypeptide is synthesized. There is no tRNA with the code complementary to a STOP signal.
The motion of mRNA is made possible by the ribosomes (purple structure in Figure 16)which are an association of proteins and a third type of RNA known as ribosomal RNA (rRNA). The larger subunit of the rRNA acts as an enzyme, speeding up the linkage of amino acids. Prior to the 1980s it was believed that only proteins could act as enzymes.
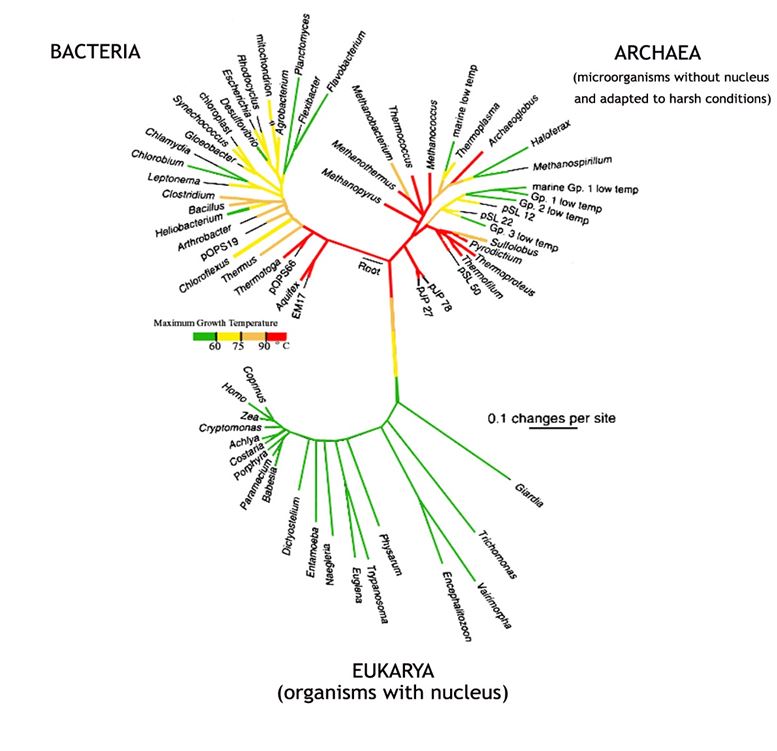
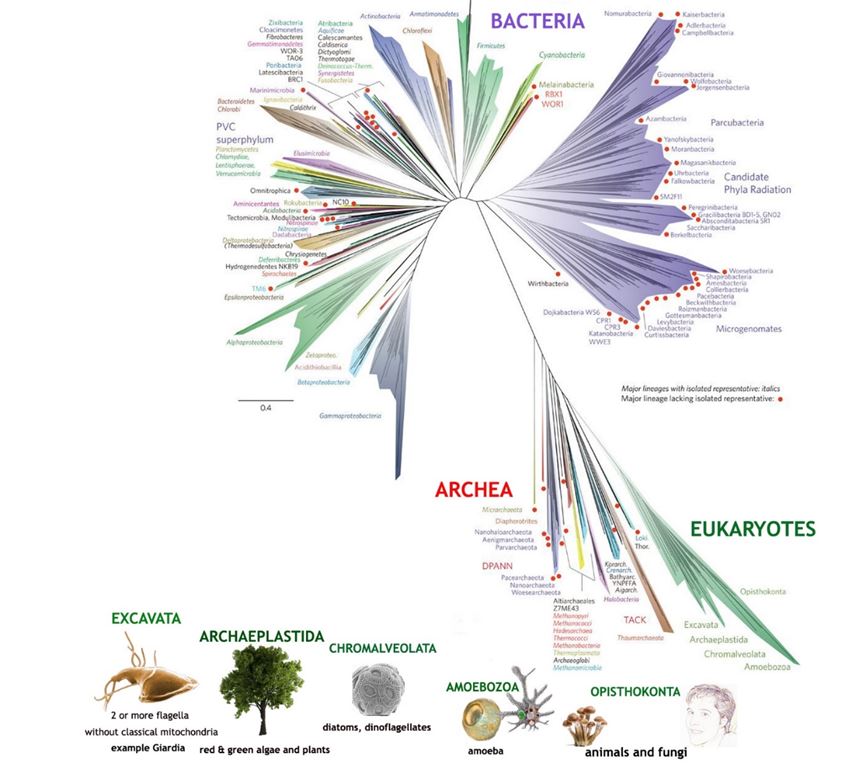
In the early 2000s the nucleotide base sequence of the small subunit RNA’s of many organisms was used as the basis of constructing an evolutionary tree (Figure 17). But this was still using a single gene as a source, so it had similar shortcomings to that of using cytochrome c as discussed in Chapter 12. A more comprehensive attempt was made in 2016, when a tree was based on the sequences of 16 different ribosomal proteins from the organisms being compared (Figure 18).
In certain viruses there is no DNA. That’s the case with ones causing the common cold, SARS, influenza, hepatitis C, Ebola and measles.For those, RNA is the only nucleic acid carrying genetic information. The same is true of a virus that does not infect people but attacks the papaya, now a target for genetic modification, to be discussed below in the next section. RNA viruses have much higher rates of mutations (changes in the genetic code) than DNA-organisms. For that reason, RNA-harboring viruses can only persist if their total number of genes is small. Their specialty is in reproducing quickly and moving on to new hosts, even new species. Due to these characteristics, natural selection has had a harder time preventing them from wiping out an entire population.
ii. Genetically modified plants, one tool among many.
Knowledge of the genetic code has given us rich insight into how life goes on while its individual carriers die. Have there been practical benefits? The papaya is a fruit native to Central America. It is a good source of vitamins C and A with an average of 61 mg and 950 IU, respectively per 100 grams of fruit and with about 2/3 of the fiber of an apple. It was the first genetically modified fruit to be grown for commercial production. It was developed in Hawaii not to tolerate herbicides or insects but to resist the papaya ringspot virus (PRSV), a type of RNA virus (Figure 19).
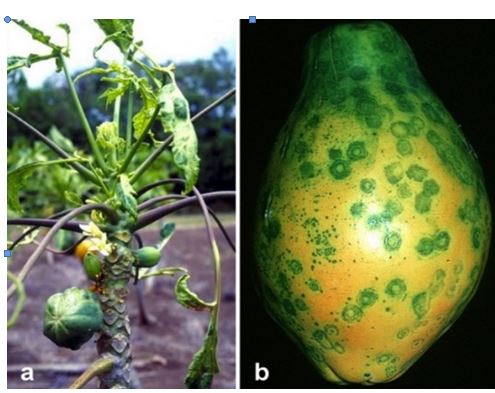
The virus was brought to the island of Oahu by tagging along with a plant in the 1930s. It either mutated into a more virulent form or another strain was brought to the island. In any case, with the help of a few species of aphids who spread the new form of the virus, papaya cultivation areas were decimated from 246 hectares in 1956 to only 16 hectares in 1968. When a plant was infected at a young age, the virus stunted its growth and it did not yield fruit. If the virus struck a mature plant, it would start yielding poor quality fruit. Puna on the Big Island of Hawaii subsequently became the major production site, but PRSV caught up to them too, reducing papaya production from 56 000 pounds in 1992 to 36 000 in 1998.
After conventional strategies failed, the key to protecting papayas from ringspot in Hawaii was to include part of the genetic material of the PRSV within papaya-DNA. From the PRSV, they isolated the RNA sequence it used to make a protein coating for itself, one of 8 proteins from a single long polypeptide. That sequence was incorporated into a ring of DNA that had two other genes, one for tracking the protein and another for switching it on. Researchers tried to get it into a callus of papaya plant tissue and across their cell walls by coating tiny beads with the DNA ring and firing them inside using a gene gun. In 1992, they took these “vaccinated” plants along with control plants and infected them all with PRSV. After a short while they found a line of plants that had incorporated the protective gene; it was the only one thriving. When tested in agricultural fields in Waimanalo, Oahu, it also showed resistance to the virus. Eventually they developed two resistant varieties, Sunup, which has two copies of the protein coat gene per cell; and Rainbow, a crossing of Sunup with a yellow flesh variety, which contains only one gene per cell. In 1998 and 2010 two other transgenic papayas were developed to resist the virus.
After seeds from resistant plant were released to farmers in 1998, overall production which had declined by 64% in the previous 6 years, improved by 20% by 2002. To this date, non-transgenic papayas continue to be grown alongside the Rainbow variety in a ratio of about one to 4 for countries that have aversions towards genetically modified products. With Rainbow in the way, the aphids don’t reach other trees as easily. In a 2011 study, no allergens or nutritional losses were found among Rainbow fruits, and only about 1% of non-transgenic papaya trees had picked up genes from Rainbow. This is because the papayas grown commercially are mostly hermaphrodites whose pollen moves from the male to female flowers of the same tree.
Following Hawaii’s success, other countries developed PRSV-resistant transgenic papaya bases on their local strains. In the same manner that there is not a single cold virus, there are different strains of PRSV. In China and Taiwan, genetically modified papayas were failing in 2018. The transgenic plants hadshown resistance initially, but they were eventually overcome by the virus as its genes recombined.
The authors of a recent review study of PRSV-resistance point out that a single solution towards the virus is not the best approach. Genetically modified papayas are just one tool among many. For example, silver reflective plastic mulches have been shown to repel aphids from young papaya plants, which delayed and reduced transmission of the virus. In Brazil they had success controlling PRSV by clearing weeds and cucurbits, a source of aphids, from the periphery of papaya orchards.
Amines
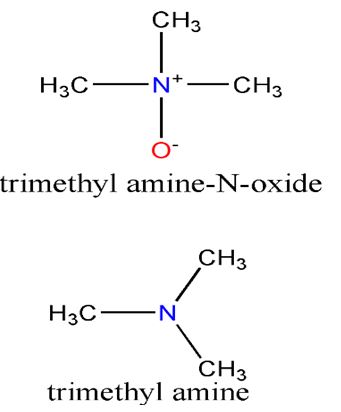
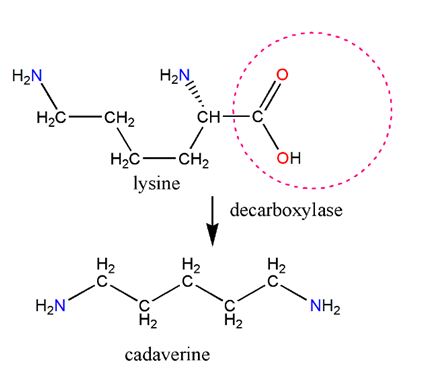
Figure 20. Biologically, cadaverine is derived from lysine. Notice, by comparing the two structures, that substituting lysine’s COOH group with hydrogen creates cadaverine. The net loss resulting from taking away COOH and adding a H is the loss of CO2, hence the name decarboxylation. Lysine decarboxylase enzyme facilitates the feat.
Few people recognize the word amine, yet just about everyone has smelled one if they have come across a dead fish or a penis that has not been washed after it has secreted semen. Amines are organic compounds that have an amino group, NH2, attached to a hydrocarbon. They are, in a sense, organic analogues of ammonia and like ammonia, most are alkaline molecules and soluble in water. Fish aroma is a complicated blend of various compounds, but a dominant one is the amine known as trimethylamine. Along with many marine organisms, fish use a similar compound, trimethyl amine-N-oxide (TMAO), to protect against the adverse effects of temperature, salinity, hydrostatic pressure and the presence of urea. When fish or other marine organisms die, bacteria quickly reduce TMAO to trimethyl amine.Given that the latter is a base, it explains why the addition of lemon juice to fish improves the taste and eliminates some of the smell. Some Newfoundlanders keep a pot of vinegar on the stove on low heat while they fry fish to attenuate the stench in their homes.
Cadaverine is another amine with an unpleasant odor, as the etymology of the word suggests. It is partly responsible for the smell of semen and decomposing flesh. It is formed by the decarboxylation of the amino acid lysine (Figure 20), and if lysine metabolism is defective, elevated levels will appear in the urine of an affected individual. There is already cadaverine in semen when it is in the prostate gland, before it is mixed with sperm cells from the testes. However, since semen also contains amino acids, it is likely that semen lingering outside the body will contain more cadaverine as lysine is converted to cadaverine by bacteria. Some chemical engineers are looking into ways of getting bacteria to produce cadaverine on an industrial scale to replace petroleum-derived hexamethylenediamine(a.k.a. cadaverine). The latter is used with adipic acid to produce Nylon.
Several neurotransmitters to be explored in Chapter 8 on potassium are amines. They include the blood-pressure-raising noradrenaline, which also acts as one of the animal’s fight or flee hormones secreted by the adrenal gland. The other, adrenaline, increases blood pressure, accelerates the heart rate lets more air into the lungs. Dopamine and serotonin.
Nitric oxide
When a single atom of oxygen and a single nitrogen interact to form nitric oxide(NO), we get a molecule with an odd sum of valence electrons. The unpaired electron makes the molecule highly reactive. Yet the compound has shown up in a variety of places, some of them highly surprising.
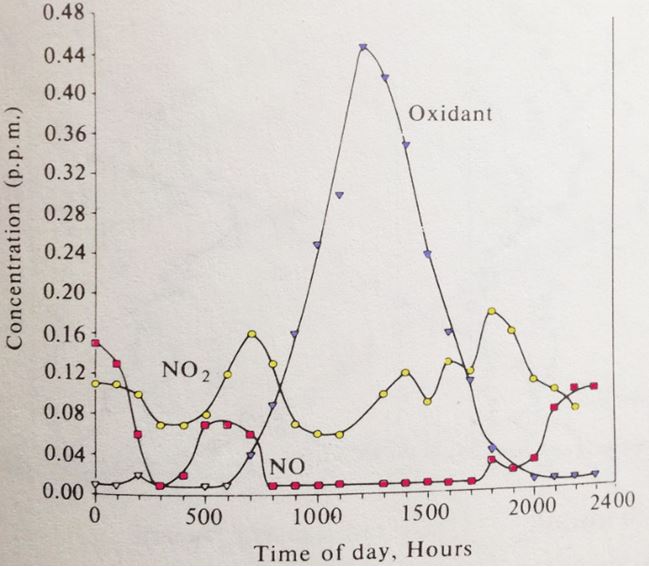
Due to nitrogen’s low reactivity, it takes a large input of energy to create nitric oxide. Intense forest fires, lightning and internal combustion engines are all capable of providing enough energy. It’s for that reason that cars are equipped with catalytic converters which take nitric oxide, theprimary nitrogen oxide pollutant, and break it back down to its constituent elements. The converters are not perfect; for vehicles that burn gasoline, 2% of the nitrogen oxides escape unscathed. For diesel vehicles their converters are only 95% efficient. With its high reactivity, any nitric oxide that escapes initiates a cascade of reactions that can lead to photochemical smog. Its formation depends on light intensity, air movement, the initial concentration of nitric oxide (Figure 21).The smog itself is a mixture of NO2, ozone and a large variety of organic compounds.
In 1998 a Nobel Prize was awarded for the discovery that nitric oxide acts as signalling molecule in the cardiovascular system. It also helps control blood pressure and blood flow by dilating blood vessels. By the time the prize was awarded, other research had revealed that nitric oxide is also a major paracrine signaling molecule in the nervous and immune systems. A paracrine signal is a hormonal effect that works only in the vicinity of the cells that secrete it. That’s related to nitric oxide’s short half life of about 10 seconds. After getting their job done, the highly reactive molecules get converted to nitrite and nitrate. When white blood cells produce larger amounts of it, nitric oxide kill microbial invaders such as harmful species of bacteria and parasites. If the immune system overreacts with the release of too much nitric oxide, its ability to dilate blood vessels backfires, blood pressure drops, and sepsis and shock could set in.
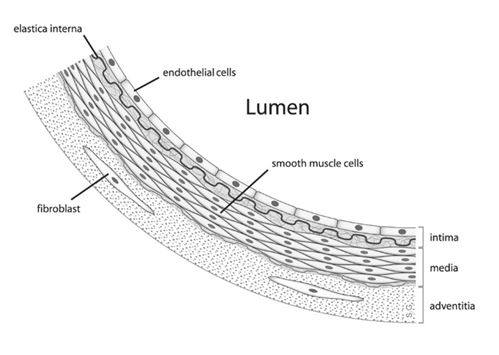
But how is nitric oxide made in the body, and how does it work as a hormone? We will use blood vessels and penile erections as an example. The story begins when, due to arousal, a nerve cell’s acetylcholine, a neurotransmitter, locks in with the receptor of cells on the interior (endothelial cells) of blood vessels. This stimulates the enzymatic conversion of the amino acid arginine to citrulline, which is accompanied by the release of nitric oxide. The NO molecules diffuse out and get into neighboring smooth muscle cells. Unlike most hormones, nitric oxide can diffuse directly across cell membranes and do not interact with a receptor. Inside a muscle cell, each NO reacts with the iron bound to the active site of the enzyme guanyl cyclase, stimulating its activity. This in turn leads to the synthesis of another molecule, cyclic guanosine monophosphate (cGMP). The presence of cGMP eventually relaxes the muscle cell by removing phosphoryl group of the muscle protein myosin’s light chain. (We will explore how muscles work at the molecular level in the phosphorus chapter). This in turn dilates blood vessels and lets in more blood flow (Figure 22). Eventually the enzyme phosphodiesterase type 5 (PDE5) degrades cGMP and the penis goes “back to sleep”.
By serendipity, a drug, sildenafil (trade name Viagra), was discovered that artificially prolonged penile erections by inhibiting PDE5, indirectly accentuating the effects of NO. A more important drug that is also related to nitric oxide is nitroglycerin. Small amounts of the explosive (0.2 to 0.5 milligrams) are given to angina patients. The drug, named glyceryl trinitrate (GTN), is eventually metabolized to nitric oxide, which dilates blood vessels and relieves the painful attack. From the Nobel Prize site nobelprize.org, we have another and classic reminder that is there is not always a clear boundary between nature, biochemical theory and industry.
Alfred Nobel invented dynamite, a product in which the explosion-prone nitroglycerin is curbed by being absorbed in kieselguhr, a porous soil rich in shells of diatoms. When Nobel was taken ill with heart disease, his doctor prescribed nitroglycerin. Nobel refused to take it, knowing that it caused headache and ruling out that it could eliminate chest pain. In a letter, Nobel wrote: “It is ironical that I am now ordered by my physician to eat nitroglycerin.” It has been known since last century (1878) that the explosive, nitroglycerin, has beneficial effects against chest pain. However, it would take 100 years until it was clarified that nitroglycerin acts by releasing NO gas.