A mineral is more pure than its parent rock. But compared to food additives, industrial compounds and pharmaceuticals, a mineral’s compound often hosts more elements. As a result it isn’t difficult to find a mineral whose atoms have a variety of cosmic origins.
Only a small percentage of elements on Earth are created in and around the planet by nuclear reactions, and even at that, they are only derivatives of atoms made elsewhere. The secondary creations result from the atmosphere’s interaction with cosmic rays, from the lithosphere’s minority of radioactive elements, from nuclear reactors and from scientific research—my favorite being the tanks that sit deep in abandoned mines collecting neutrinos from our sun and supernovae.

pezzottaite
So where in space did the majority of constituents of the living-geological continuum originate and by what mechanism? Let’s look at the cosmic roots of the six elements of a mineral known as pezzottaite, discovered in Madagascar and only officially recognized as a distinct mineral in 2003. Its formula is Cs(Be2Li)Al2Si6O18
Oxygen
What’s the ultimate source of oxygen? Big or small, stars spend the bulk of their time on the main sequence, a hydrogen-fusing stage that actually lasts longer for smaller stars. This is because a star’s lifetime is proportional to its mass but inversely proportional to the fourth power of its core temperature. Although small stars have less hydrogen, the smallest of the chemical elements, they also fuse it at a lower temperature from the lower force acting on its core. The product of the sequence of reactions involved in the fusion of hydrogen is helium. While helium grows as an onion-like outer-layer during its residence as a main sequence star, the temperature isn’t high enough to fuse the helium into bigger elements. But when the hydrogen fuel runs out, the star is for a while no longer in equilibrium. The outward radiative pressure isn’t there to balance out gravity, so the large force towards the star’s center “ignites” the fusion of helium and the star becomes a red giant.
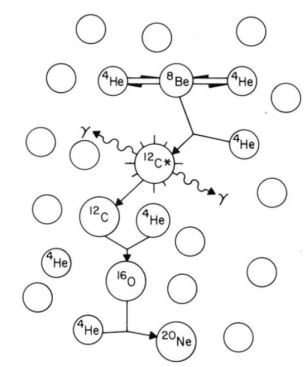
from J. Chem. Educ., 1990, 67(9), p 726
When the star’s core temperature reaches 108 K, from the diagram we see a pair of helium nuclei fusing to form an unstable beryllium nucleus, which then fuses to give us the life-essential carbon. This in turn fuses with another helium to produce oxygen. Oxygen can continue to fuse, but there are enough nuclei that remain as such. When stars, in a later stage of their evolution, either shed their outer layers either as a planetary nebula or supernova, stellar dust receives these oxygen atoms, some of which ended up in our water , skin and in our pezzottaite.
Silicon
To get silicon we need a more massive star capable of generating a red-giant-temperature and density of 500 million K and 5 million g/cm3. Under these conditions two oxygens (atomic number 8) will combine to create a silicon nucleus(atomic number 16). Fittingly some of this product and progenitor are eventually reunited on planets as sand, sandstone, quartz, clay and a wide variety of minerals that contain either silica or some form of silicate— including that of pezzottaite.
Lithium and Beryllium
A neat thing about pezzottaite is that it has two(lithium and beryllium) of three light elements that are relatively rare in the universe. The presence of each of lithium, beryllium and boron is only one billionth that of hydrogen and about a millionth of that of carbon, nitrogen and oxygen. The reason for this is that the bulk of Li, Be and B do not survive any of the stages of stellar evolution. Their fragile nature suggested they were synthesized in low-density, low-temperature environments.

The broken line represents solar system abundance of the elements Li, Be and B. The solid line shows enrichment found in galactic cosmic rays. From J Chem Ed 1990, 67(9)p 729
One area where a high concentration of these light elements occurred was in galactic cosmic rays. This suggested that perhaps they are not being carried from elsewhere but being synthesized on the spot by the nuclear reaction between alpha particles(helium nuclei) or protons of the cosmic rays and larger elements like carbon, nitrogen and oxygen. This process is now called spallation. While other genesis-models failed to predict the exact concentrations of the isotopes, the spallation hypothesis came closest to account for the relative ratios.

scan from scientific American May 1987, from an article by researchers Viola and Mattheson
In the 1980s Viola and Mattheson used a cyclotron to accelerate protons and helium nuclei to the energies of cosmic rays and aimed them at targets of He, N, C and O to generate new nuclei. When the particles’ energies and speeds were analyzed, their calculated masses allowed them to identify the isotopes. Their abundance was similar to that found in galactic cosmic rays. The three reactions that created a fair amount of the lithium and beryllium in our pezzottaite are:
4He + 12C → 7Li + 2 4He + 1H (main isotope of lithium, 92.5% of what’s found on Earth)
2 4He → 6Li + 1H + 10n
1H+ 14 N →9Be + 4He + 2 1H
One anomaly, however, was that the amount of 7Li ( the heavier isotope) made in the cyclotron was a little lower than what’s actually found in space, suggesting that a minority of 7Li was not made in the cosmic rays but originated elsewhere. Some of the discrepancy is partly accounted for by the small amount made in the Big Bang, but in 2013, analyses of the Subaru Telescope High Dispersion Spectrograph revealed that a more significant contribution comes from novae. Smaller stars eventually become white dwarfs after passing through the red giant stage. But these remnants, if part of a binary system, could suddenly brighten from explosive nuclear reactions when material from its partner-star is pulled onto the dwarf’s surface. The nuclear reactions create a different series of elements compared to those produced in stellar interiors or during supernova explosions. One of these atypical reactions is the conversion of beryllium-7 to lithium-7 by electron capture, which lowers the atomic number without affecting the atomic mass.
Cesium
To explain the origin of the final two elemental components of pezzottaite, Al and Cs we need to examine supernovae. The next avenue of evolution of large stars is a type II supernova, which briefly outshines its entire galaxy. When all fuel is spent in large stars iron is left at the stellar core and a gravitational collapse ensues. In a rapid process-set of reactions ( r-process), neutrons are initially generated by the gravitational collapse during photodisintegration, a process where gamma causes the fission of heavier nuclei. For example here’s a sequence of reactions generating a total of 7 neutrons from a single iron nucleus:
56Fe + ϒ → 13 4He + 4 10n
4He + ϒ → 2 ‘H + 2 10n
‘H + e- → 10n + ν
Then in the actual r-process neutrons are captured to form unstable neutron-rich isotopes which then undergo beta decay and turn into elements of higher atomic number.How does this happen? A little background info: Being electrically neutral, neutrons can penetrate the positively charged nucleus, especially at low temperatures. But free-roaming neutrons are short-lived lasting only about ten minutes as one of their down-quarks becomes an upquark, a process that generates a proton, a beta particle and an antineutrino. This raises the atomic number by 1. To generate sufficient numbers of neutrons and provide a constant supply of these ephemeral neutral particles, the high-energy environment of something like a supernova is needed. For example with the provided energy, gamma will break down enough iron to generate enough neutrons, which in turn can convert other iron atoms (atomic number 26) into heavier iron isotopes, one of which will beta-decay into cobalt (number 27). That isotope of cobalt can then absorb more neutrons and eventually undergo beta decay to create an even higher-numbered element, Ni. The isotopes created by the r-process are not the stable ones of the heavier elements. But they can later become stable ones by undergoing fission and beta decay.
Once the stellar material has been enriched with the ejection of these new atoms, subsequent generations of stars can generate other isotopes in the slow process (s -process), which also involves absorption of neutrons but at a slower rate. In a less violent environment such as that of a red giant, the absorbed neutron has time to decay into a proton so it tends to produce isotopes of medium to lower atomic numbers. For example some of pezzottaite’s cesium 133 (atomic number 55) could have been directly produced by the breakdown of an isotope created by the r-process or it could have formed later in another generation of stars by the beta-decay of xenon 133:
13354 Xe →133 55 Cs +0 -1 β
As shown in the diagram below, the unstable xenon 133 isotope was in itself generated by s-process. A 5-step sequence of neutron-absorption beginning with xenon-128 took place. In an r-process environment, more neutrons would have been absorbed before beta decay would have been possible.

Illustration of the r and s processes operating in the vicinity of cesium’s neighbors. each square is a stable isotope, like that of 133 Cs. The horizontal solid arrows represent neutron capture, while the wavy diagonal arrows represent beta decay. The isotopes represented by white boxes result from either the s or r process. The blue boxes represent isotopes that result only from the r process, while the red boxes are s-only isotopes. The yellow boxes represent isotopes produced by proton capture. from https://answersingenesis.org/astronomy/solar-system/discussion-stellar-nucleosynthesis/
Aluminum
Finally we get to aluminum. Before becoming a type II supernova, there is an important set of reactions that occurs in the core of a star exceeding 8-11 solar masses. Silicon burning -reactions mainly begin with silicon(atomic number 14) and add on a helium nucleus, creating sulfur(16), argon(18), and so on until iron is formed. But above a critical temperature explosive silicon burning photodisintegrates all nuclei and rebuilds them up during the expansion. In one of these reactions magnesium-26 captures a proton to form aluminum 27.
William Blake was right. There is indeed a world in a grain of sand—and, we may add, a universe in a mineral.
Sources:
Formation of the Chemical Elements and the Evolution &
of Our Universe, V. E. Viola Journal of Chemical Education, 1990, 67 (9)
Scientific American Grant J. Mathews, Victor E. Viola May, 1987
Click to access thielemann.pdf
http://subarutelescope.org/Pressrelease/2015/02/18/index.html
Click to access thielemann.pdf
http://www.aanda.org/articles/aa/full_html/2013/11/aa22599-13/aa22599-13.html
http://adsabs.harvard.edu/full/1971ApJ…166..153A
https://answersingenesis.org/astronomy/solar-system/discussion-stellar-nucleosynthesis/
https://en.wikipedia.org/wiki/Silicon-burning_process
http://abyss.uoregon.edu/~js/ast122/lectures/lec18.html
http://pages.uoregon.edu/jimbrau/astr122-2009/Notes/Exam2rev.html






 Now we observe. As we stated at the onset, many snowflakes land in the same place. But only a few meters above any given spot, it is apparent that many paths lead to a common destination. Some flakes tumble; some abandon the terminal velocity we took so long to calculate, and they yield themselves to whimsical eddies. How they arrive is influenced not only by shape, mass and gravity but by sheer luck—luck due to the random, pinpoint fluctuations in temperature and pressure that affect their air space.
Now we observe. As we stated at the onset, many snowflakes land in the same place. But only a few meters above any given spot, it is apparent that many paths lead to a common destination. Some flakes tumble; some abandon the terminal velocity we took so long to calculate, and they yield themselves to whimsical eddies. How they arrive is influenced not only by shape, mass and gravity but by sheer luck—luck due to the random, pinpoint fluctuations in temperature and pressure that affect their air space.





