
An old picture of the author’s daughter floating in a sea of ripening tomatoes in her grandmother’s garage.
Tomatoes have to be ripe to make a good sauce. Many of these compounds only form during the ripening process. But if you just squeeze ripe tomatoes, you will get tomato juice and not sauce. If tomatoes are cooked , even more compounds will be created, changing the taste of the juice, but it will still have an unappealing flavor. To get the full aroma and mouth-watering taste we need a non-random orchestra of compounds consisting of organic acids, soluble sugars, amino acids, pigments and over 400 compounds capable of interacting with our nasal receptors. This translates into a need for more food ingredients.
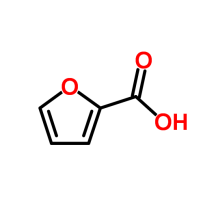
Furoic acid, sometimes called hydroxy furfural is the basic structure of similar molecules that contribute to the pleasant smell of sauteed onions
While tomatoes are being blended with herbs and carrots, a separate ingredient, the onion, is sautéed to further enhance the sauce’s flavor. Why? Onions are about 4% sugar with a glucose to sucrose to fructose ratio of 2:1:1. The high pan-heat is absorbed by the oil, which transfers some of the energy to the cut onions. The sucrose decomposes to fructose and glucose, temporarily adding to what’s already present. Then some of the latter pair of molecules dehydrate or go through other means of decomposition to yield pleasantly aromatic molecules such as butane-2,3 dione and several derivatives of furoic acid. Meanwhile other glucose and fructose molecules bond to produce a variety of oligomers responsible for the brown colour and unique taste of sautéed onions. What we have described is the process of caramelization, which is still not fully understood.
After letting the mixture of cooked onions and oil cool for five minutes, we place it into the blender with the carrot, herbs and tomatoes. Why? By doing so, the products of caramelization will mix more evenly with the bulk of the sauce, and we will also be creating a mixture that will at least be temporarily homogeneous.

(A) Oil-oil interactions are weaker than those between water and oil (B) For this reason oil spreads into a thin layer when added to water. (C) But unless the droplets are small enough and/or stabilized by a third party molecule, stronger water-water interactions prevent oil from mixing thoroughly.
Since the bonds between water molecules are stronger than those between water and oil, stirring oil and water does not create a thoroughly consistent mixture. But high speed-blending will break up the oil from the cooked onion into small droplets. Water will interact with these droplets but without abandoning them, water molecules will still be capable of forming hydrogen-bond interactions with their own kind. In other words we will have an emulsion.

Two emulsions. The one on the left has no emulsifier. On the right the emulsifier is adsorbed on the surface and its two types of interactions reduce surface tension.
- Unraveling the Chemical Composition of Caramel . Journal of Agricultural Food Chemistry. School of Engineering and Science, Jacobs University Bremen, 28759 Bremen, German
- Molecular Gastronomy. Hervé This. 2008
- Nutrient analysis from : https://ndb.nal.usda.gov/ndb/foods/show/3223?manu=&fgcd=&ds=COOKING
- Physical Chemistry. Laidler, Keith. Maiser, John. 2002
- https://themoleculargastronomyadventure.wordpress.com/tag/caramelan/

