- If it wasn’t for my atypical stellar atmosphere, I would be of no value to astronomers. I have a layer of ionized hydrogen and fully ionized helium, neither of which can lose energy to outer space. So these ions keep absorbing energy from my interior until I expand. This eventually lowers my density and allows electrons to recombine with ions and neutralize them. Now excitation can lead to loss of energy to outer space, eventually decreasing radiative pressure. This makes me shrink. Hydrogen and helium then reionize, and the cycle repeats itself.
- Since temperature is related to luminosity, there are more ionized atoms associated with more luminous stars of my type. And the more there are, the longer it takes to go through a cycle. In chemistry, a correlation of concentration and color becomes more practical if it relies on prepared standard solutions of known concentrations. Similarly if it wasn’t for parallax measurements for nearby relatives, the relationship between luminosity and the logarithm of periods could not be calibrated.
- A technique known as spatial scanning has allowed the Hubble Space telescope to extend the range of measurements for my type of star.

By applying spatial scanning to astronomical parallax, NASA’s Hubble Space Telescope can be used to make precision distance measurements 10 times farther into our galaxy than previously possible. Image source: NASA/ESA, A.Feild/STScI
- Spectroscopy lets astronomers determine whether I am metal-rich or not. Each of the two types has a different linear relationship.
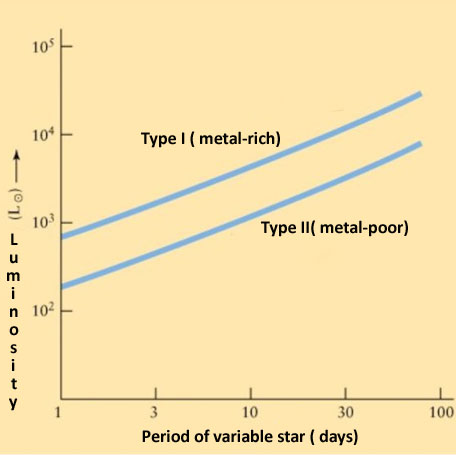
The same period can correspond to different luminosities, depending on whether this type of variable star is metal- rich(Type I) or metal poor. A metal-rich star is a star that has a higher proportion of any element other than hydrogen or helium, even though the elements may not necessarily be metals from a chemical standpoint.
- So thanks to my star-type, astronomers have a way of measuring the distance of galaxies. Using these distances from spatial scanning and thanks to Hubble’s infrared camera, astronomers could correct the apparent brightness of my star-type to the values that would be observed if they all were located at a standard distance of 10 parsecs (1 parsec = 3.26 light years). When the observed brightness is plotted against the periods of various variable stars of my type, , the ratio of the brightness for the corrected curve to that of the galaxy in question is determined. The reduction in brightness factor is then square rooted because of the inverse square relationship between the distance of a star and its brightness. If the denominator of that result is then multiplied by ten parsecs, we get the distance to that galaxy or star cluster. Here’s an example involving the Large Magellanic Cloud.

The blue data is for a group of variable stars whose brightness has been adjusted for a distance of 10 parsecs from the earth. The red data represents data for the brightness of variable stars in the Large Magellanic Cloud, which are 4940^2 dimmer. From this we can conclude that the Large Magellanic Cloud is 4940 *10 parsecs from the earth or 49400*3.26 =160 000 light years away. Source: http://hubblesite.org/hubble_discoveries/science_year_in_review/pdf/2006/cepheid_calibration.pdf
- Probably the best known star of my type is Polaris. It has a period of only four days. In contrast, the period of RS Puppis is about 40 days.

RS Puppis is one of the brightest stars of its type in the Milky Way Galaxy. Picture source: https://www.spacetelescope.org/news/heic1323/ The source describes how a light echo was used to determine its distance.
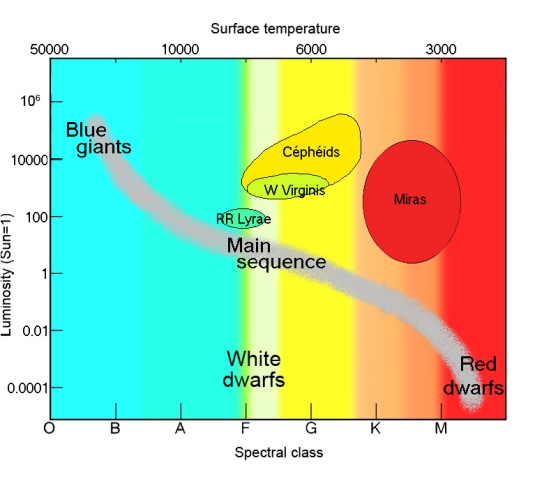
- In the Hertzsprung-Russell diagram, I am on an instability strip.
For more What Am I blogs, see:
uvachemistry.com/2014/08/07/what-chemical-compound-am-i/
uvachemistry.com/2016/07/11/again-guess-whats-being-described
uvachemistry.com/2016/06/22/guess-whats-being-described
Sources:
Universe. William J. Kaufmann II. W.H. Freeman
NASA Cepheid Calibration http://hubblesite.org/hubble_discoveries/science_year_in_review/pdf/2006/cepheid_calibration.pdf









