Someone can observe a fair number of sunrises without becoming jaded. There is often an unprecedented observable variation in the pattern and thickness of clouds, which changes the corresponding array of colours. Add to it the sense of scale and the feeling of rejuvenation with every dawn, and each sunrise has the potential of inspiring a line like Cat Stevens’ “Morning has broken like the first morning”.

So far I have only walked through Arizona’s Petrified Forest once, but I cannot imagine being jaded from repeated hikes. Almost every log of stone appears unique, and its perception, as every good photographer realises, is influenced by lighting conditions. Touching them is not monotonous either. In some petrified wood, the ends of the logs are rugged; in others the mineral has been cleaved, as if by a blade. In others, the ridges and the grain of the original tree are preserved.
And what intensifies the sense of wonderment is the mechanism that created these masterpieces of natural history. Remarkably the first rigorous laboratory study was only published recently in May of 2016. As the authors Marisa Acosta and George Mustoe pointed out, prior to their research, explanations of petrified wood’s colour were derived from an unpublished analysis of limited rock samples and from speculation.

The story of Arizona’s petrified wood started about 225 million years ago during the Late Triassic period, when all land was part of a splitting Pangaea, when dinosaurs and large reptiles dominated. Flowering plants had not yet evolved, so there were no poplars, maples or palo verdes, Arizona’s national tree. Cacti, also being angiosperms, had not arisen either, and they would not have lived there anyway because the land within present-day Arizona was then a lush subtropical forest filled with the ancestors of present day conifers. In one area, these clung to eroding riverbanks and the trees fell into streams. Buried in flood plains, they were out of contact with oxygen. Anaerobic conditions slowed their decomposition. That in itself would not have been sufficient for petrification (the process of turning into stone without seeing Medusa’s head 🙂 . There was a lot of volcanic ash nearby, which introduced silica into the ground water,probably in the form of Si(OH)4. With the cell walls still intact, their cellulose and lignin (components of wood) had an affinity for silica. How the silica(quartz) formed chemically from Si(OH)4 is a mystery so far because the process has not been replicated in the lab.
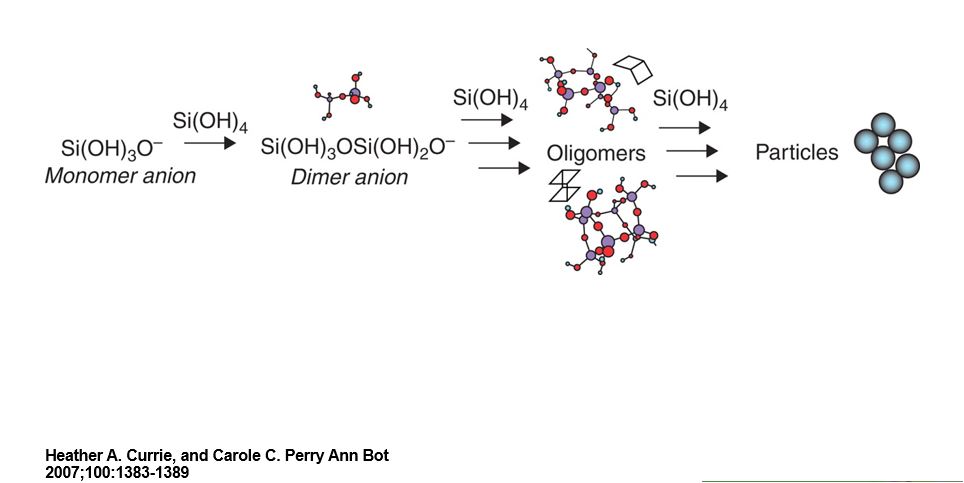
Then the intracellular spaces also filled with the same quartz mineral. But there wasn’t just one single episode of permineralization. As we shall see, a given sample’s varied oxidation states of iron in the silica suggest that the solidifying wood was exposed to penetrating solutions exposed to different environmental conditions over the course of time.
It was previously believed that ions of copper, chromium, manganese and aluminate were responsible for the assortment of colours in petrified wood. But the authors collected over 30 samples from 3 sites in Arizona, including the Petrified Natural Park and also from Nevada, Oregon and Zimbabwe. Using a petrographic machine they prepared 200 um slides and then used Laser Ablation Inductively Coupled Plasma Mass Spectrometry(LA-ICP-MS) to identify the key impurity colouring the silica. The technique is highly sensitive in identifying elements directly on solid samples. Using LA-ICP-MS they began by focusing a laser beam on each thin rock sample to generate fine particles. The ablated particles were then transported to the secondary excitation source of the ICP-MS instrument for digestion and ionization of the sampled mass. The excited ions in the plasma torch were finally sent to a mass spectrometer detector for elemental analysis.
Rainbow patterns in petrified wood especially were believed to be caused by a variety of metal ions. But iron alone was the key to the hues of red, purple and yellow. Strangely iron and not chromium was also responsible for the green colour in 1/3 bright green samples and in ¾ of dark green rocks. It’s already known that both abiotic and biotic pathways can reduce the +3 state of iron in ferric oxyhydroxide minerals to a +2 state to produce green rust or fougerite( Fe2+ 4 , Fe 3+ 2 (OH)12 CO3.3 H2O). Those of course are the actual minerals involved in the green parts of petrified wood. But the fact that the different oxidation states of iron in red and green minerals coexist in the same rock sample suggests that permineralization is not a one-shot deal. The following graph reveals how combinations of pH and exposure to the atmosphere influence the electron-ripping (oxidation)or electron-donating abilities (reduction) of solutions. The latter is measured by Eh, a solution’s oxidation potential. As the Eh changes over time, it could use different events to precipitate a different colour in the same log, even if iron is involved in both cases.

One will also notice abstract art–like patterns in the petrified wood. The authors describe a number of physical processes at work. These include diffusion, dilution, and a form of natural chromatography. The black parts, contrary to the previous speculation, are not due to the presence of manganese, as revealed by the analysis. Total absorption of light by the silica structure could be one cause, or in some cases small amounts of persistent organic matter were responsible.
The Petrified Forest is in essence a nature-made museum of art, history and geochemistry.
